NURS 6053 Alterations In Cellular Processes
 This document aims to explore the pathology of cystic fibrosis (CF), drawing on both scientific understanding and personal experiences with a friend who has the condition.
This document aims to explore the pathology of cystic fibrosis (CF), drawing on both scientific understanding and personal experiences with a friend who has the condition.
The topics covered include the cellular origins of CF, the role of genetics in its manifestation, the rationale behind the specific symptoms presented by CF patients, an exploration of the cells involved in this disease, and how various factors (e.g., gender, genetics) might alter a child’s response to cystic fibrosis.
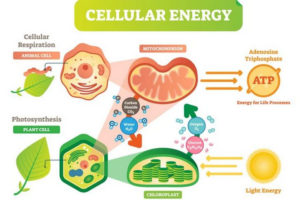
At its core, cystic fibrosis is a genetic disorder caused by mutations in the CFTR gene, located on chromosome 7. These mutations interfere with the normal transport of ions across cell membranes, leading to dehydration within cells and the production of thick, dry secretions.
The Role of Genetics in Cystic Fibrosis
The genetic foundation of cystic fibrosis is crucial in understanding its pathophysiology. As noted in the article Cystic fibrosis genetics: from molecular understanding to clinical application, cystic fibrosis is an autosomal recessive genetic disorder. This means that for an individual to manifest cystic fibrosis, they must inherit two copies of the mutated CFTR gene, one from each parent. The CFTR gene encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein, which plays a critical role in regulating chloride and sodium ion movement across cell membranes (Cutting, 2015). When mutations occur in this gene, the CFTR protein does not function properly, which results in the thick mucus and other complications characteristic of the disease.

Struggling to meet your deadline?
Get your assignment on NURS 6053 Alterations In Cellular Processes done by certified MDs and PhDs in the USA. ORDER NOW!
Why the Patient is Presenting with the Specific Symptoms Described
Cystic fibrosis manifests through a variety of symptoms, many of which are linked to the malfunctioning CFTR protein. In the case of the patient described, the symptoms of salty skin, abdominal swelling, and inadequate weight gain are indicative of CF’s effects on multiple organ systems, particularly those composed of epithelial tissues such as the sweat glands, pancreas, and gastrointestinal tract.
According to the Journal of Cystic Fibrosis article “Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy,” the thick mucus obstructs the airways, leading to chronic respiratory infections, while the loss of pancreatic function contributes to malnutrition due to the inability to properly absorb nutrients. This is why enzyme replacement is often required to aid digestion and prevent the development of cellular blockages due to undigested nutrients (Cutting, 2015). 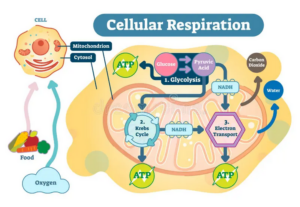
The Physiologic Response to the Stimulus and Why This Response Occurred
The underlying pathophysiology behind the symptoms described involves mutations in the CFTR gene, which lead to a defective CFTR protein. As the CFTR protein is responsible for chloride transport across cell membranes, a mutation in this gene causes a reduction in chloride ion movement, which disrupts water and salt transport. This leads to thick, sticky mucus in various organs. The symptoms of salty skin, abdominal swelling, and poor weight gain are a direct result of this altered cellular process. Specifically, the altered ion transport leads to water absorption being skewed, which affects the ciliary function in the lungs, making it difficult to clear mucus. In the gastrointestinal tract, the disruption in sodium, chloride, and potassium reabsorption leads to blockages that prevent proper nutrient absorption, which explains the child’s growth and nutrition issues (McCance et al., 2019).
The Cells Involved in This Process
The primary cells involved in cystic fibrosis are epithelial cells. These cells line the respiratory tract, pancreas, liver, sweat glands, and reproductive organs. As mentioned in the article by Soleti et al. (2013), dysfunction in these epithelial cells, particularly in the CFTR protein, results in defective ion transport, leading to cellular dehydration and the production of thick mucus. The continuous inflammation in the epithelial cells of affected organs leads to cellular apoptosis (programmed cell death) as a consequence of ongoing stress and injury to the tissue. This inflammation ultimately contributes to the progressive damage observed in cystic fibrosis, including lung damage and pancreatic insufficiency.
How Another Characteristic (e.g., Gender, Genetics) Would Change Your Response
Genetics plays a fundamental role in the transmission of cystic fibrosis, as the disease is inherited in an autosomal recessive pattern. If both parents are carriers of a mutated CFTR gene, their child has a 25% chance of inheriting two mutated copies and developing cystic fibrosis. If the child inherits only one mutated copy, they become a carrier but typically do not exhibit symptoms. However, the severity of the disease can vary depending on the specific mutations present in the CFTR gene. Some mutations result in milder forms of CF, while others may lead to more severe symptoms (Montoro et al., 2018).
Gender also plays a role in the expression of cystic fibrosis. Males with CF often experience more severe reproductive complications, such as infertility due to the absence of the vas deferens, a common manifestation of the disease (McCance et al., 2019). While gender differences may not drastically change the symptoms of cystic fibrosis, they can influence aspects of the disease, such as reproductive health or the overall severity of lung damage.
Conclusion
Cystic fibrosis is a complex genetic disorder that primarily affects the function of epithelial cells across several organ systems. Understanding the genetic basis of cystic fibrosis, its cellular mechanisms, and how these lead to specific symptoms is crucial for effective patient care. As practitioners, it is important to advocate for early screening and regular checkups to ensure early diagnosis and management of CF symptoms. Providing parents with information about cystic fibrosis, including genetic testing and symptom monitoring, can help ensure better outcomes for children with this condition. Early intervention is key to preventing complications and improving quality of life for patients with cystic fibrosis.
References
Cantin, A.M, Hartl, D, Konstan, M and Chmiel, J. (2015). Inflammation in Cystic Fibrosis Lung Disease and Therapy. Journal of Cystic Fibrosis. Retrieved from https://www.cysticfibrosisjournal.com/article/S1569-1993(15)00058-2/fulltext
Cutting G. R. (2015). Cystic fibrosis genetics: from molecular understanding to clinical application. Nature reviews. Genetics, 16(1), 45–56. https://doi.org/10.1038/nrg3849
McCance, K. L. & Huether, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children (8th ed.). St. Louis, MO: Mosby/Elsevier.
Montoro D.T., Haber A.L., Biton M. et at. (2018) A Revised Airway Epithlial Heiracrchy Inclues CFTR-Expressing Inoncytes.Nature. DOI: 10.1038/s41586-018-0393-7
Soleti, R., Porro, C., & Martínez, M. C. (2013). Apoptotic process in cystic fibrosis cells. Apoptosis : an international journal on programmed cell death, 18
NURS 6053 ALTERATIONS IN CELLULAR PROCESSES
 This case study examines a 27-year-old patient with a history of substance abuse who was found unresponsive by emergency medical services and subsequently regained responsiveness following naloxone administration. The analysis explores the role of genetics, the mechanisms underlying the patient’s symptoms, the physiological response, the cells involved in these processes, and the influence of other characteristics, such as age, on the patient’s condition and treatment.
This case study examines a 27-year-old patient with a history of substance abuse who was found unresponsive by emergency medical services and subsequently regained responsiveness following naloxone administration. The analysis explores the role of genetics, the mechanisms underlying the patient’s symptoms, the physiological response, the cells involved in these processes, and the influence of other characteristics, such as age, on the patient’s condition and treatment.
The Role of Genetics in Opioid Use Disorder
Genetics play a significant role in the development of substance use disorders, particularly opioid use disorder (OUD). Specific genetic variations have been shown to increase susceptibility to addiction, particularly in individuals who may have a family history of substance abuse. The OPRM1 gene (which codes for the mu-opioid receptor) and its A118G variant are known to influence an individual’s response to opioids and may predispose certain individuals to opioid dependence (Crist et al., 2019). Additionally, other genes such as OPRD1 (which codes for the delta-opioid receptor), KCNG2, CNHIH3, KCNC1, RGMA, and APBB2 have been implicated in opioid use disorder, particularly in individuals of European descent (Liu et al., 2019). These genetic factors can contribute to a heightened risk of addiction by altering how the brain responds to substances like opioids, affecting both the rewarding effects of the drug and the individual’s susceptibility to developing dependence.
The Patient’s Symptoms and the Underlying Physiology
The patient’s unresponsiveness was caused by central nervous system (CNS) depression resulting from an opioid overdose. Opioids, such as heroin or prescription painkillers, work by binding to specific receptors in the brain and spinal cord, which suppresses both pain and respiratory function. In overdose situations, this leads to profound CNS depression, respiratory depression, and a loss of consciousness. Naloxone, an opioid antagonist, was administered, which successfully reversed the effects of the opioid overdose by displacing the opioid molecules from the receptors, thereby restoring normal respiration and consciousness.
After the reversal, the patient experienced pain in the left hip and forearm, which can be understood as the rebound effect following opioid cessation. Opioids not only relieve pain but also mask underlying discomfort. When the opioid effects are reversed, pain that was previously suppressed becomes more pronounced. Additionally, the patient exhibited signs of tissue necrosis, which could be attributed to poor tissue perfusion caused by CNS depression. This impaired circulation may have resulted in hypoxia in tissues, leading to cell death.
Furthermore, the patient developed respiratory acidosis due to the initial opioid overdose, which decreased the ability of the lungs to expel carbon dioxide (CO₂). This led to an increase in serum potassium levels, causing hyperkalemia and changes in the electrocardiogram (ECG), which can be dangerous if left untreated (Parthvi et al., 2019). Hyperkalemia can result in arrhythmias or even cardiac arrest, which is why prompt treatment is necessary.
The Cells Involved in the Patient’s Symptoms
The key cells involved in this patient’s symptoms include mast cells, neutrophils, and macrophages, which play central roles in the body’s response to tissue injury and pain. Mast cells are involved in the release of histamines and other inflammatory mediators that initiate pain and inflammation. Neutrophils are the first responders to tissue damage, while macrophages help in both initiating and resolving inflammation. These immune cells work together to mediate the inflammatory response, which is vital to healing but can also contribute to pain perception and tissue damage.
Additionally, adaptive immune cells, such as T cells and B cells, are involved in the longer-term immune response and contribute to the chronic pain process. These immune cells play a role in managing the body’s pain response and the resolution of inflammation, which could be disrupted in the case of opioid overdose and its reversal.
The Impact of Age on the Patient’s Response and Treatment
Age is an important characteristic to consider when evaluating the physiological response to opioid overdose and subsequent treatment. In older adults, the sensitivity to pain stimuli may be altered, and they may experience a reduced response to opioids due to changes in receptor function or drug metabolism. However, older individuals may also have more comorbidities (such as cardiovascular disease or kidney dysfunction) that can complicate both opioid overdose and treatment, making age a crucial factor in the management of opioid use disorder and overdose.
For example, in elderly patients, an opioid overdose might present with less pronounced symptoms due to the blunted pain response, but the risk of complications like respiratory depression or cardiac arrhythmias may be higher. Therefore, treatment must be tailored based on age and underlying health conditions, as older patients may not metabolize or clear opioids as efficiently as younger individuals, which can prolong the effects of both the drug and its reversal agent, naloxone.
Conclusion
In summary, this case highlights the complex interplay of genetics, opioid use disorder, and the physiological responses to opioid overdose and naloxone administration. The genetic predisposition to addiction, along with the mechanisms of opioid action on the CNS, led to the patient’s unresponsiveness, which was successfully reversed by naloxone. The rebound pain, tissue necrosis, and changes in potassium levels highlight the physiological consequences of opioid use and overdose. Additionally, the patient’s age is a critical factor that influences the response to opioids and treatment interventions, emphasizing the need for personalized care in opioid overdose situations.
References
Crist, R. C., Reiner, B. C., & Berrettini, W. H. (2019). A review of opioid addiction genetics. Current Opinion in Psychology, 27, 31–35. https://doi.org/10.1016/j.copsyc.2018.07.014
Liu, M., Jiang, Y., Wedow, R., Li, Y., Brazel, D. M., Chen, F., Datta, G., Davila-Velderrain, J., McGuire, D., Tian, C., Zhan, X., Choquet, H., Docherty, A. R., Faul, J. D., Foerster, J. R., Fritsche, L. G., Gabrielsen, M. E., Gordon, S. D., Haessler, J., … Vrieze, S. (2019). Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nature Genetics, 51(2), Article 2. https://doi.org/10.1038/s41588-018-0307-5
Machelska, H., & Celik, M. Ö. (2020). Opioid Receptors in Immune and Glial Cells—Implications for Pain Control. Frontiers in Immunology, 11. https://www.frontiersin.org/articles/10.3389/fimmu.2020.00300
Parthvi, R., Agrawal, A., Khanijo, S., Tsegaye, A., & Talwar, A. (2019). Acute Opiate Overdose: An Update on Management Strategies in Emergency Department and Critical Care Unit. American Journal of Therapeutics, 26(3), e380. https://doi.org/10.1097/MJT.0000000000000681
I agree with your insights regarding the diagnosis of Group A Streptococcus (GAS) infections and the role of cellular injury induced by bacterial pathogens. Cellular injury can arise from various stimuli, including immune responses, nutritional deficiencies, and disruptions caused by pathogens. In this discussion, I will explore the role of nutritional imbalances in cellular injury, and how excess or deficiency of essential nutrients can contribute to pathological conditions, including chronic diseases like atherosclerosis.
Cellular Injury Due to Nutritional Imbalances
Cellular injury can result from a range of internal and external factors. Nutritional imbalances are one key contributor to cellular dysfunction. Cells depend on a variety of macronutrients (proteins, carbohydrates, fats) and micronutrients (vitamins, minerals, trace elements) to maintain proper structure and function. When there is an insufficient supply of these nutrients, cells cannot perform essential functions such as energy production, protein synthesis, or membrane integrity. This can lead to cellular stress and damage (McCance & Huether, 2019). 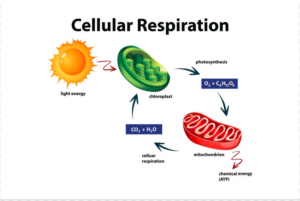
For instance, protein deficiencies may impair cellular repair mechanisms, while micronutrient shortages (such as vitamin C or zinc) can hinder immune responses and increase the vulnerability to infections. Moreover, insufficient fatty acids or carbohydrates can compromise cell membrane integrity and cause dysfunction in signaling pathways, which can ultimately lead to cell death or inflammation.
Excess Nutrients and Cellular Damage
While deficiencies in nutrients are a common cause of cellular injury, nutrient excesses can also be damaging. When cells are exposed to an overload of nutrients, such as lipids or glucose, it can lead to lipid accumulation and metabolic disturbances. The excessive intake of macronutrients, such as fats and carbohydrates, can lead to insulin resistance, increased oxidative stress, and inflammation, all of which are associated with cellular injury and tissue damage.
Hyperlipidemia, a condition characterized by elevated levels of lipids in the blood, is one example of how nutrient excess can contribute to disease. Research by Xu et al. (2023) demonstrated that hyperlipidemia is a significant factor in the development of atherosclerosis, a condition where fatty deposits build up in the arterial walls, leading to narrowed arteries and reduced blood flow. This pathological process is thought to occur when excessive lipids and other substances such as cholesterol accumulate in the endothelial cells of the blood vessel walls, resulting in inflammation, oxidative stress, and the eventual formation of plaques that impede circulation.
Atherosclerosis is a key example of how nutrient overload can foster the development of chronic conditions by damaging cellular structures and initiating a series of pathological events that ultimately affect the cardiovascular system. The imbalance between pro-inflammatory molecules (released by immune cells) and anti-inflammatory factors in the vascular walls leads to chronic disease progression.
The Intricate Relationship Between Cellular Health and Chronic Disease
The connection between cellular health, nutritional balance, and the risk of chronic disease underscores the complexity of maintaining proper cellular function. Nutritional imbalances, whether due to deficiencies or excesses, can disrupt normal cellular processes and contribute to long-term pathological conditions such as atherosclerosis, diabetes, and obesity. The damage caused by nutrient imbalances can activate inflammatory pathways and promote oxidative stress, further exacerbating the cycle of cellular injury and disease development.
A key takeaway from the relationship between nutritional imbalances and cellular health is that the body requires a delicate balance of nutrients to maintain optimal cellular function and prevent the onset of chronic diseases. Understanding how both deficiencies and excesses contribute to cellular injury provides important insights into potential preventive measures and therapeutic interventions.
Conclusion
In conclusion, cellular injury is not only influenced by external factors such as pathogens but also by internal factors, particularly nutritional imbalances. Both nutrient deficiencies and excesses can result in cellular damage, disrupt normal cellular processes, and contribute to the development of chronic diseases such as atherosclerosis. The delicate balance of nutrition is crucial for cellular health and underscores the importance of maintaining proper dietary habits to prevent chronic diseases. Research into these mechanisms offers important clues for preventive strategies and therapeutic interventions aimed at reducing the burden of conditions related to poor cellular health.
References
McCance, K. L. & Huether, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children (8th ed.). St. Louis, MO: Mosby/Elsevier.
Xu, N., Ijaz, M., Shi, H., Shahbaz, M., Cai, M., Wang, P., Guo, X., & Ma, L. (2023). Screening of Active Ingredients from Wendan Decoction in Alleviating Palmitic Acid-Induced Endothelial Cell Injury. Molecules, 28(3), 1328. https://doi.org/10.3390/molecules28031328Links to an external site.
Hello Courtney,
I appreciate your thorough and insightful analysis of the patient’s scenario. Your recognition of the genetic predisposition to substance abuse is indeed crucial, as genetic factors have a significant impact on an individual’s susceptibility to addiction and response to drugs. Additionally, your understanding of the patient’s symptoms and their potential causes is well-aligned with current clinical knowledge. In this response, I will further explore the role of genetics in substance abuse, the pathophysiology behind the patient’s reported symptoms, and the clinical significance of tissue necrosis and hyperkalemia in the context of opioid use and overdose.
Genetic Predisposition to Substance Abuse
As you rightly pointed out, genetic factors contribute significantly to an individual’s vulnerability to substance use disorders (SUD), including opioid use disorder (OUD). Variations in specific genes, such as those associated with drug metabolism (e.g., CYP450 enzymes) and dopamine receptors (e.g., DRD2), can influence how drugs are processed in the body, thereby altering the risk of addiction or overdose (Young, 2019). For example, certain genetic variants may lead to a faster metabolism of opioids, requiring higher doses to achieve the desired effect, which increases the risk of overdose. Conversely, some genetic variations may lead to slower metabolism, causing drug accumulation and increasing the potential for adverse reactions.
Additionally, the presence of genetic predisposition can affect how individuals respond to treatment interventions, such as naloxone administration, and may influence treatment adherence and outcomes in addiction recovery. This underscores the importance of considering genetic factors when developing personalized treatment plans for patients with substance abuse issues.
Tissue Necrosis and Inflammatory Response
The patient’s symptoms, specifically the burning pain over the left hip and forearm, strongly suggest local tissue necrosis at the injection sites. As you mentioned, injection drug use often leads to tissue damage, infections, and the formation of abscesses, which can result in necrotic tissue. Injection drug use, particularly intravenous use, introduces foreign substances directly into the bloodstream, and can lead to local inflammatory responses at the site of injection, as well as systemic complications like sepsis (Usach et al., 2019). The burning pain the patient reported is likely indicative of this inflammation and tissue destruction at the injection sites.
Additionally, rhabdomyolysis, a condition in which muscle tissue breaks down due to ischemia or trauma, can occur when there is severe tissue damage from drug use, leading to muscle cell death and the release of intracellular contents (such as myoglobin) into the bloodstream. These byproducts can be harmful to the kidneys, leading to acute kidney injury (AKI) and electrolyte imbalances such as hyperkalemia, which we will discuss next.
Hyperkalemia and Electrocardiogram (EKG) Findings
Your recognition of the prolonged PR interval and peaked T waves on the EKG as signs of hyperkalemia is astute. Hyperkalemia, an elevated level of potassium in the blood, is a common consequence of rhabdomyolysis and acute kidney injury (AKI). When muscle cells break down, potassium is released into the bloodstream, leading to electrolyte imbalances that can significantly affect cardiac function (Usach et al., 2019). Peaked T waves and a prolonged PR interval are classic EKG findings associated with hyperkalemia, which can lead to arrhythmias, including ventricular fibrillation or asystole, if not addressed promptly.
The combination of tissue necrosis, rhabdomyolysis, AKI, and hyperkalemia presents a life-threatening situation for the patient, as the imbalance in electrolytes can compromise cardiac conduction and renal function, both of which are critical to maintaining homeostasis. As you mentioned, the careful monitoring of potassium levels and kidney function, as well as prompt intervention, such as IV fluids and medications, is essential for managing these complications.
Clinical Implications and the Need for Individualized Treatment
Your analysis underscores the complexity of the case and highlights the need for a comprehensive clinical evaluation. Each patient’s presentation may involve a unique set of factors, including genetic predisposition, the extent of substance abuse, and the presence of comorbidities such as infections or renal dysfunction. This variability emphasizes the importance of an individualized approach to patient care.
As healthcare providers, it is crucial to consider all aspects of the patient’s health history, including their genetic profile, substance use patterns, and the specific pathophysiological mechanisms at play in each case. This allows for the development of tailored treatment plans that address the patient’s immediate needs (e.g., managing overdose and electrolyte imbalances) while also considering long-term goals, such as addiction recovery and preventing recurrence of overdose or other complications.
Conclusion
In summary, the patient’s symptoms and clinical presentation reflect the intricate interplay between genetics, substance abuse, and the pathophysiological consequences of overdose, including tissue necrosis, rhabdomyolysis, and hyperkalemia. Your analysis effectively demonstrates the importance of considering the patient’s genetic factors, the local and systemic effects of drug use, and the electrolyte disturbances in managing opioid overdose and related complications. This highlights the need for a comprehensive, individualized treatment approach that addresses both immediate and long-term patient needs.
References
Usach, I., Martinez, R., Festini, T., & Peris, J. E. (2019). Subcutaneous injection of drugs: literature review of factors influencing pain sensation at the injection site. Advances in therapy, 36, 2986-2996.
Young, A. I. (2019). Solving the missing heritability problem. PLoS genetics, 15(6), e1008222.

Dont wait until the last minute.
Provide your requirements and let our native nursing writers deliver your assignments ASAP.